As the landscape of 340B program compliance continues to evolve, staying ahead of reimbursement challenges and legislative shifts is crucial for covered entities. In this white paper, Kristin Fox-Smith explores proactive strategies to navigate the complexities of 340B contract pharmacy arrangements, manage ESP data effectively, and respond to ongoing legislative changes.
Legislative Landscape and Opportunities
The 340B program faces significant challenges due to evolving state legislation and manufacturer restrictions, particularly concerning contract pharmacies. This has led to noticeable erosion in the manufacturer space concerning reimbursement and restrictions on contract pharmacies. 340B covered entities are experiencing difficulties maintaining revenue streams and ensuring compliance with evolving regulations. Despite these hurdles, there are exciting trends and opportunities, particularly in states that have enacted protective legislation or are considering such measures, which offer potential for growth and improved reimbursement.
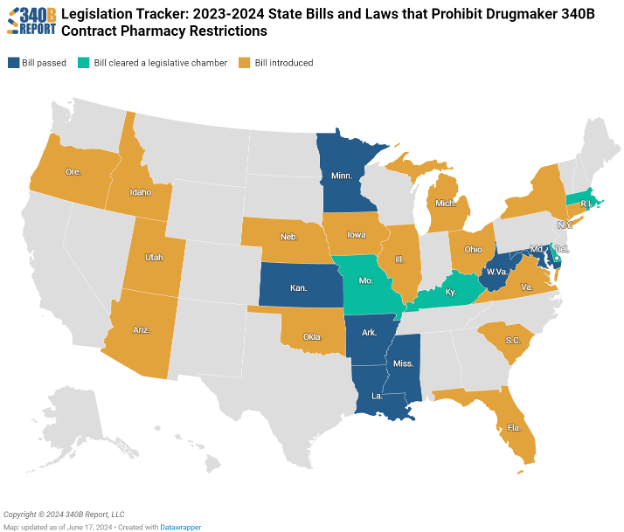
Currently, both Arkansas and Louisiana have rapidly implemented laws protecting 340B pricing. This swift action has ensured that reimbursement remains unaffected by the 340B status of entities and provides a blueprint model for other states.
Following their lead, West Virginia and Mississippi are in the process of enacting similar legislation, which bodes well for the future. Additionally, Kansas and Maryland are on the brink of passing comparable bills, indicating a positive trend. Virginia has also shown recent legislative progress, suggesting that further beneficial changes are on the horizon.
States with enacted legislation offer critical protection for 340B entities. These protections ensure that reimbursement remains stable regardless of 340B status – which is essential for the financial stability of covered entities. While the pace of legislative change varies, ongoing efforts in other states aim to replicate these protections, all but guaranteeing a more secure future for 340B.
*Several states, such as Arkansas, Louisiana, West Virginia, and Mississippi, have taken steps to protect 340B pricing and reimbursement, setting a precedent for others to follow. Courtesy of 340B Report: Contract Pharmacy Protection Bill Map – 340B Report.
Specific Manufacturer Updates and Data Submission Requirements
Arkansas and Louisiana:
The Federal appellate court upheld an Arkansas law prohibiting drug manufacturers from restricting 340B drug discounts for providers using contract pharmacies. Louisiana’s law prevents drug companies from denying or restricting the acquisition or delivery of drugs under the federal 340B drug pricing program at contract pharmacies. For covered entities in Arkansas and Louisiana, accurate and timely data submission is crucial. Claims data must be submitted within 45 days of medication dispense to retain access to 340B pricing at contract pharmacies.
Manufacturer-Specific Restrictions:
- Merck: Effective April 12, 2024, restrictions apply primarily to hospitals and consolidated health centers. Contract pharmacies wholly owned by a 340B covered entity or health system are no longer exempt from these restrictions. For entities in Arkansas and Louisiana, uploading claims data to the 340B ESP portal is essential to maintaining access to 340B priced replenishment at contract pharmacies.
- Astellas: Effective May 1, 2024, 340B covered entity hospitals, outside Arkansas and Louisiana, must not designate contract pharmacies if they have in-house outpatient pharmacies.
- AbbVie: Effective April 17, 2024, hospitals with in-house outpatient pharmacies that are not part of AbbVie’s limited distribution network (LDN) can designate one of the LDN pharmacies within 40 miles of the parent covered entity and must submit claims within 45 days of medication dispensing.
- Genentech: Restrictions apply to hospital 340B covered entities with in-house outpatient pharmacies. Submission of claims to 340B ESP is voluntary but crucial for maintaining compliance.
- Sumitomo: Applies to all 340B covered entities, with specific rules for in-house and wholly owned pharmacies. Limited distribution drugs, like Orgovyx, must be designated to one qualified pharmacy within the LDN network, and claims must be submitted within 45 days.
Managing ESP Data: Best Practices
Effective data management is fundamental for maximizing 340B program benefits. This involves ensuring data accuracy, timely submission, and proper utilization of the ESP portal. To achieve this, covered entities must adopt several best practices.
First, data accuracy and timeliness are paramount. Submitting claims data accurately and within required timeframes helps avoid inquiries and potential denials. It is crucial to maintain up-to-date data that reflects legislative and manufacturer changes. Second, the regular update and management of the ESP portal are essential. This ensures that the portal reflects current pricing and designation statuses, aligning with the latest data requirements. Third, strategic designation of contract pharmacies must be performed annually, taking into account volume and financial viability. Utilizing in-house pharmacies effectively and ensuring proper designation for specialty and non-specialty drugs can significantly enhance program benefits.
Strategic Considerations for Covered Entities
Given the complexity and variability of manufacturer restrictions, it is imperative for covered entities to stay informed and proactive. Here are key strategic considerations:
- Annual Pharmacy Designation: Entities must review data carefully to designate the most viable pharmacy locations annually, ensuring financial viability and compliance.
- Specialty Pharmacy Designations: Special attention should be given to specialty drugs requiring separate designations. This flexibility can be advantageous in optimizing pharmacy networks.
- Wholly Owned Exemptions: The exemption for wholly owned contract pharmacies being lifted opens new channels for reimbursement, enhancing the financial landscape for covered entities.
- Data Submission Accuracy: Timely and accurate data submission to the 340B ESP portal is critical. Errors or delays can lead to inquiries or loss of 340B pricing, making diligent data management essential.
Conclusion
Navigating the evolving 340B landscape requires a proactive approach – focusing on effective data management, strategic designation of contract pharmacies, and robust legislative advocacy. By implementing these strategies, covered entities can maximize reimbursement, ensure compliance, and continue to provide essential care to those who need it most.
Kristin Fox-Smith, 340B ACE, is Managing Director with Hospital and Health Systems at Visante. Providing innovative, expert solutions that help covered entities reach their 340B program compliance goals and optimize their programs for long-term success, Visante’s 340B ACE consultants are available to help staff or manage programs and are fully dedicated to the client’s success. Learn more at visanteinc.com, or reach out to solutions@visanteinc.com or 866-388-7583.