Maintaining a successful 340B program demands attentiveness and continued optimization. In this white paper, Draffin Tucker’s Brittany Edwards delves into critical considerations and best practices for effective account maintenance in the 340B landscape. From self-audits to crosswalk management, Edwards explores key strategies to ensure compliance, maximize savings, and uphold program integrity – equipping covered entities with the necessary tools to navigate the complexities of the 340B program with confidence.
Strengthening Your Program with Proactive Self-Audits
Conducting regular self-audits is vital to the health of your 340B program. These audits act as a diagnostic tool to identify and address compliance discrepancies and to streamline program operations for better efficiency. It’s crucial to critically evaluate your self-audit processes, pinpoint any areas where non-compliance has occurred, and implement sustainable corrective measures. Don’t just apply quick fixes; dig deep to uncover and resolve underlying systemic problems that may pose risks for future compliance breaches. A thorough and proactive self-audit strategy is essential for maintaining the integrity and effectiveness of your 340B program.
Enhancing Program Integrity with Rigorous Data Feed Oversight
Careful monitoring of dispense data feeds will help you quickly identify anomalies that may signal compliance risks. Whether dispense data is managed by a third-party administrator or through tracking modules, it’s essential to know the data sources for your program. Periodically review data for red flags, check distances, and verify encounter feeds for eligibility to avoid using encounters from ineligible locations.
Avoiding Duplicate Discounts & Ineligible Dispenses
Root out instances of duplicate discounts and ineligible dispenses by continuously reviewing your dispense data. Determine whether your entity bills Medicaid fee-for-service for 340B purchase drugs (carving in) or not (carving out) and conduct self-audits across all payer types to identify and rectify duplicate discounts. It’s also helpful to review Medicaid billing forms and ensure accurate NPIs to safeguard against inadvertent revenue loss.
Maintaining Your Crosswalk
If your covered entity uses a third-party administrator or split billing software, maintain timely updates in your crosswalks to accurately match purchases with dispenses. Pay attention to drug details to prevent errors in matches and avoid unnecessary purchases on non-340B accounts.
Identifying Incorrect BUPP/UOI/Multipliers
It’s essential to routinely refresh your system’s crosswalks, paying particular attention to medications that have the most impact on your budget. Monitor your billing units per package (BUPP), units of inventory (UOI), and multipliers with diligence to avoid the pitfalls of overstocking or underordering, which could lead to unnecessary expenditure or outstanding dues to drug manufacturers. A collaborative review of these critical figures is key to accurate replenishment calculations, ensuring your 340B program remains economically sound and operationally effective.
Reviewing Filters and Qualification Rules
Continually assessing your qualification rules and filter settings is a strategic approach to strike the perfect equilibrium between cost-efficiency and regulatory adherence. It’s about finding that sweet spot where expanding your filters could mean enhanced savings without stepping into non-compliance territory. Before you adjust these settings, take a comprehensive look at the advantages they bring against the potential risks. Thoughtful adjustments here can lead to significant improvements in your program’s performance while maintaining full compliance with 340B program requirements.
Implementing Targeted Random Auditing
Implementing targeted random audits is a powerful tactic to intensify the impact of compliance checks. It’s not just about selecting random samples; it’s about smartly choosing areas that are more prone to discrepancies, such as transactions involving providers not recognized on the official list, prescriptions filled beyond the tenure of provider contracts, or those with unusually high or low dispense volumes. By honing in on these potential red flags, you can better deploy your resources, ensuring that they make the biggest difference where it counts, thereby bolstering the robustness of your 340B program.
Enhancing Program Efficiency with Strategic Referral Oversight
Thorough examination of referral patterns serves a dual purpose: it ensures you’re capturing all eligible expenses and helps streamline your audit process by reducing unnecessary reviews. By putting in place strong referral tracking systems, you not only shore up the efficiency of your program but also bolster your cost-saving measures. This strategic focus on referral scrutiny allows you to direct your resources judiciously, maximizing the financial health and sustainability of your 340B program.
Streamlining Procurement with Diligent Purchase-Dispense Analysis
An ongoing comparison between your 340B drug purchases and patient dispenses is critical for pinpointing any misalignments that could indicate diversion or reconciliation issues. Take a closer look at medications that show a high volume of purchases but lack corresponding dispensing records. Understanding the root causes of these mismatches is vital. It provides clear guidance for making smarter procurement choices and can lead to more effective cost management. Regularly conducting such detailed analyses is key to refining your purchasing strategy and reinforcing the cost-effectiveness of your 340B operations.
Maximizing Savings Through Strategic Drug Spend Analysis
Delving deep into the spending trends of your drug purchases can uncover valuable opportunities to refine your 340B acquisition strategy and cut down on costs outside the program. By examining your purchasing data, particularly through pivot tables, you can spotlight the drugs that are driving the most expense in both your 340B and non-340B categories. Dissect the factors contributing to any non-340B purchases and address these to tighten your spending. This level of detailed analysis is crucial for optimizing savings and ensuring that your 340B program is as cost-effective as possible.
Aligning Policies with Practice for Enhanced Compliance
It’s imperative to guarantee that your 340B program’s policies and procedures are not just theoretical guidelines but are also reflective of day-to-day operations. Engage in regular revisions of your policy documents to ensure they are up-to-date and accurately mirror the practical aspects of your program’s execution. This alignment is crucial to avoid any inconsistencies during audits and to maintain a seamless operational flow. By keeping your policies in lockstep with your practices, you strengthen the program’s compliance framework and integrity.
Maintaining Accuracy and Compliance through OPAIS Vigilance
Routinely verifying the details of your covered entity and contract pharmacies in the Office of Pharmacy Affairs Information System (OPAIS) is a crucial step for maintaining 340B program compliance. It’s essential to confirm the accuracy of addresses, contact details, and other pertinent information listed in OPAIS. A meticulous approach to reviewing OPAIS records not only ensures that all information is current and complete but also positions your entity for successful recertification. This proactive oversight is a key part of sustaining your program’s compliance and securing its ongoing eligibility.
Empowering Decision-Making with Custom Data Dashboards
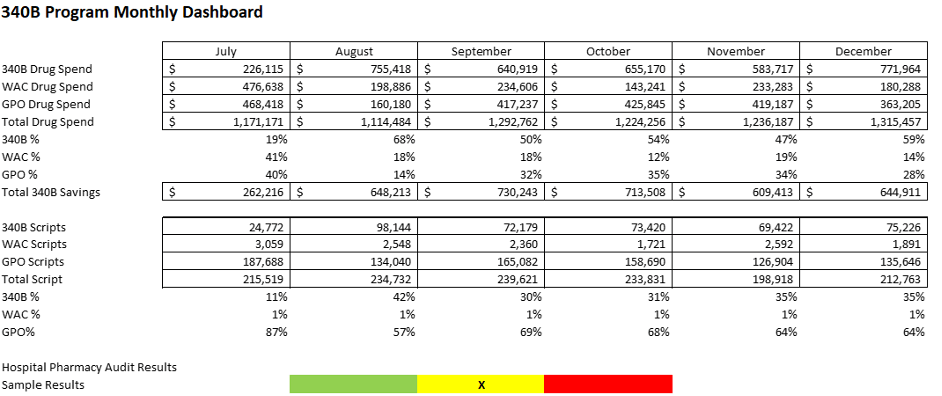
The use of customized, data-driven dashboards is instrumental in transforming raw data into strategic insights. By tailoring these dashboards to reflect your specific metrics, you provide stakeholders with a powerful tool that brings clarity to the program’s performance. This heightened visibility fosters informed decision-making, allowing you to react swiftly to trends and make adjustments that drive your 340B program towards its goals. These dynamic dashboards are vital for stakeholders to gain a comprehensive understanding and take evidence-based actions. See example dashboards below.
Example 1
Example 2
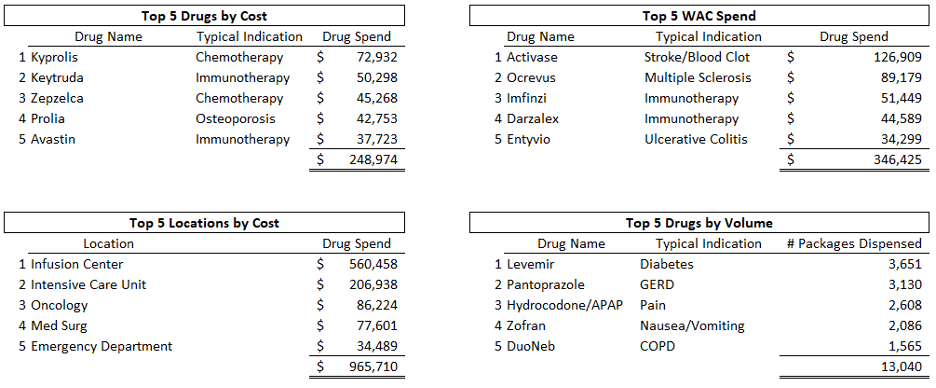
Strategically Managing Manufacturer Restrictions
Effectively handling manufacturer restrictions requires a proactive and deliberate approach. Consistently monitoring drug prices is essential to remain compliant with manufacturer guidelines and to avoid unintended financial setbacks. By electing a singular contract pharmacy location, you can simplify the procurement process and ensure adherence to 340B price conditions. Moreover, the careful application of third-party administrator (TPA) filters plays a crucial role in mitigating risks associated with the inadvertent purchase of drugs at non-340B prices, particularly during periods of price volatility. These strategies are key to navigating the intricate landscape of manufacturer restrictions successfully.
Cultivating a Culture of Continuous Improvement
The cornerstone of sustaining a successful 340B program lies in fostering habits that drive success and ensure enduring growth. Regular audits are the backbone of this process, providing a framework for consistent compliance and the optimization of financial benefits. Staying abreast of industry developments through webinars, conferences, and targeted communications keeps you informed and ready to adapt to emerging trends. Networking with fellow 340B professionals is invaluable, as it opens doors to a wealth of collective knowledge and shared experiences that can shed light on common obstacles and innovative solutions. Finally, nurturing a culture of curiosity and open dialogue by actively asking questions is fundamental to deepening understanding and refining your program. These habits are not just actions but investments in the future of your 340B program, ensuring it remains resilient, responsive, and aligned with the ever-evolving 340B landscape.









